Old How
The following guide was designed to help organizations decide how to implement routine tumor screening for Lynch syndrome in order to maximize success.
On this page
- Making the Case for Lynch Syndrome Tumor Screening and Engaging Stakeholders
- Planning How to Implement Tumor screening for Lynch Syndrome
- Optimizing and Maintaining Tumor Screening
Making the Case for Lynch Syndrome Tumor Screening and Engaging Stakeholders
Making the case for Lynch Syndrome Screening
- Provide screening recommendations:
- Show published evidence that tumor screening is cost effective.
- Make a case for how screening identifies cases that are/were being missed at your own or other institutions – without routine screening only 3% of individuals with Lynch syndrome are identified
- Share studies showing that nearly all patients favor tumor screening. [ADD LINKS]
- Create a list of other institutions conducting tumor screening in your area or show them a list of LSSN member institutions.
Engaging Stakeholders
Engaging all stakeholders early on will help everyone feel ownership and ensure “buy in”. However, you may wish to use this guide to create a tentative plan then obtain feedback from other key stakeholders such as:
- pathologists
- genetic counselors
- geneticists
- administrators
- gastroenterologists
- GI oncologists
- colorectal surgeons
- gynecologic oncologists
Find one or more champions: Champions include people who will make this a priority. Those in a leadership position and people whose opinion other key stakeholders value may also be helpful.
Ways to educate and engage
- Hold a conference on Lynch syndrome tumor screening (provide continuing education credits)
- Devote time to Lynch syndrome during tumor board meetings
- Use LSSN introductory information and PowerPoints
- Include a persuasive story about the positive effect screening can have for patients and their family (preferably a true story from your own institution)
- Elicit any barriers to implementing screening at your own institution and arm yourself with information for overcoming barriers.
- Send out an initiation letter announcing implementation of screening
Planning How to Implement Tumor screening for Lynch Syndrome
Which Tumor Screening Procedures to Use
Who to screen
- In 2009 a CDC working group called the Evaluation of Genomics Application in Practice and Prevention (EGAPP) made an evidence-based recommendation in favor of universal tumor screening (UTS) [i.e., screening all newly diagnosed colorectal cancer patients for Lynch syndrome].
- National Comprehensive Cancer Network (NCCN) guidelines recommend universal screening for all newly diagnosed colorectal and endometrial cancers to identify Lynch syndrome and simplify the process.
- Limiting tumor screening to a subset of patients is referred to as criterion based screening (CBS) and has proven to be much more complex than UTS
| UTS considerations | CBS considerations |
|---|---|
| Fewer patients will be missed if all patients are automatically screened UTS is more expensive than CBS, but there are several studies reporting UTS is cost-effective Pathologists or clinicians won’t have to remember the Bethesda or other criteria | CBS will identify more patients than if no routine screening were performed, but fewer than UTS [an estimated 25-75% of patients are missed depending on the screening criteria used]. Screening a subset of patients is less costly than UTS The process is harder to automate because physicians or pathologists are left to determine which tumors to screen unless there is a way to automatically flag the samples in your electronic medical record Pathologists do not typically know or have access to the family history in order to apply all of the Bethesda criteria |
Which specimens to screen (biopsy vs. surgical resections)
| Biopsy considerations | Surgical resection considerations |
|---|---|
| Enables surgical decision-making (subtotal vs. segmental resection) Rectal tumors have not been exposed to neoadjuvent chemo & RT yet so IHC is more reliable than after neoadjuvent chemo Often not enough tumor or normal tissue to do MSI Screening could be done twice (once on biopsy and once on surgical resection) thereby decreasing cost effectiveness Patient may be lost to follow-up if they don’t have surgery or have surgery elsewhere | Cannot inform surgical decision-making Rectal tumors with neoadjuvent chemo & RT could have false absence of MSH6 Can perform MSI and/or IHC Ensures test is only done once Patient is less likely to be lost to follow-up |
Who orders the screen
- Setting up a system so screening is automatically done on all tumors has been described by some pathologists as the easiest approach for them and it saves them time.
- If the screening needs to be ordered, there is always the possibility that cases will be missed (less likely if there is some way to flag criteria in electronic medical records).
What method of screening (IHC vs. MSI)
- There is insufficient evidence to conclude that one method is better than the other
- May determine which to use based on institutional resources available
- Some studies suggest IHC is less expensive
- IHC requires more skill to interpret accurately
- The potential increase in patient identification from using both MSI and IHC on all tumors may not justify the added cost from a public health screening standpoint
Automatic reflex testing using hypermethylation or BRAF testing
- Approximately 15% of sporadic tumors demonstrate microsatellite instability (MSI), usually due to MLH1 promoter hypermethylation; and this results in absence of the MLH1 and PMS2 proteins on IHC. Consequently, these individuals will have a positive tumor screen even though they do NOT have Lynch syndrome.
- Additional screening of all MSI-high tumors or those tumors with absence of MLH1 and PMS2 can help distinguish individuals with sporadic cancer from those who likely have Lynch syndrome.
- Additional screening reduces the number of individuals who need to be seen by genetics for counseling and germline genetic testing and therefore it can save patients time and potential worry.
- Unless patients have other risk factors for hereditary cancer, those who have hypermethylated tumors or the presence of a BRAF V600E mutation need no further testing because it is unlikely they have Lynch syndrome.
| Hypermethylation testing | BRAF testing |
|---|---|
| Hypermethylation should be used for endometrial tumors and can be used for colorectal tumors. In the absence of other risk factors, no further testing is needed if the tumor is hypermethylated. Other risk factors warrant germline panel testing (e.g., germline testing is typically offered to all those with colorectal cancer under age 50. | BRAF can be done for colorectal tumors, but NOT endometrial tumors. BRAF only identifies ~2/3 of CRC tumors with hypermethylation. However, BRAF testing may be more feasible if institutions already have it available. When the BRAF V600E mutation is found in a colorectal tumor no other testing is needed unless other risk factors are present. |
Screening and informed consent
- Institutions DO NOT require explicit informed consent for tumor screening. However, several patients have expressed how they would like some information about tumor screening at the time of diagnosis.
- Consent is typically needed before doing germline genetic testing to confirm a diagnosis of Lynch syndrome.
Planning for Successful “Screen Positive” Results Follow-up
Documenting abnormal tumor screening results
- When a tumor is MSI-high or when IHC finds absence of one or more mismatch repair proteins there is an increased chance the patient has Lynch syndrome. When additional screening is conducted on MSI-high tumors or those with absence of MLH1 and PMS2, it is considered a “positive screen” only if a BRAF V600E mutation is NOT found or if there is NO evidence of MLH1 promoter hypermethylation.
- All patients with a positive tumor screen should be offered genetic counseling and germline testing to confirm if they have Lynch syndrome.
- Several centers reported results getting lost in medical records or never being reported to patients.
- Screen positive results should be entered into the pathology report and flagged in the electronic medical record if possible.
- Sample reports can be found here.
Ensuring patients with positive screening results are offered genetic counseling and germline testing
- Patients with a positive screen will need information about germline testing in order to make an informed decision. Patients can either receive a full pre-test genetic counseling session or a shorter informed consent session with plans for a full genetic counseling session once results of germline testing are available.
- To ensure screen positive patients are offered genetic counseling and testing to confirm a diagnosis of Lynch syndrome, several institutions reported the need for results to be sent to a designated person who takes responsibility for ensuring that the patients and/or treating physicians are aware and follow-up.
- Several institutions have found higher patient follow-up rates when one or two people are responsible for results tracking.
- When patients cannot be reached, it may be useful to send a letter to the patient and/or reminders to the treating providers. Sample letters for screen positive patients can be found here [ADD LINK].
- Having a genetic counselor (GC) or trained nurse in genetics disclose results and indicate they are calling on behalf of the patient’s treating physician has been helpful for some institutions because:
- They already know about Lynch syndrome and deal with this routinely.
- This takes the burden off physicians who have several other competing demands and may be unable to prioritize spending time discussing genetic implications.
Improving Patient Follow-up with Counseling and Germline Testing
- At centers with the highest levels of patient follow-up, GCs or genetic nurses meet in-person with the patient immediately before or after the patient meets with the treating physician as part of a routine follow-up appointment.
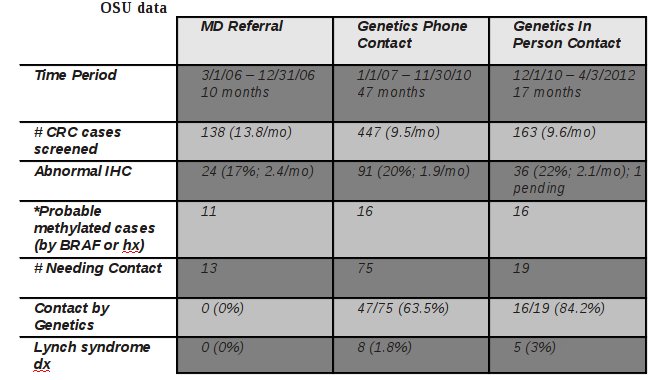
- This is not always possible, but the experience at OSU and Cleveland Clinic [LINK TO article] provide data to support the value of this approach.
- Some centers that are moderately successful with patient follow-up have genetic counselors remind treating physicians which patients need follow-up and physicians subsequently stress to the patient the importance of germline testing to confirm Lynch syndrome.
- Other ways to increase follow-up may be to:
- Eliminate any need for the patient to be referred to genetics or automating the referral and scheduling process can help
- Schedule GC follow-up to coincide with another follow-up visit
- Have all healthcare providers involved in patient care stress the importance of follow-up
- Make patients aware of available funds for germline testing of uninsured patients who meet certain qualifications, or any other funding that may be available to ensure patients can access germline testing
- Try again later to reach patients who have not followed through because they may have fewer competing demands or feel a bit less overwhelmed after treatment
- Send a letter to the treating physician to remind them to follow-up with the patient and/or to send a referral (included in implementation section)
- Communicate during tumor boards to remind treating physicians of which patients still need to follow-up
- Put an electronic reminder system in place
Planning for “Screen Negative” Results Follow-up
- Negative screening results should be documented in the chart. However, simply putting results in the chart means that the opportunity to catch patients at high risk for other hereditary cancer syndromes may be lost.
- Having an active tracking mechanism or someone review basic information about patients with negative results can identify others for whom a genetics referral is appropriate. This improves quality of care and has resulted in the diagnosis of other hereditary cancer syndromes.
- Some centers send a standardized result letter to all screen negative patients. (See implementation section). This is useful in case they are asked about this in the future and it also provides an opportunity to explain that there may be other causes of hereditary cancer and encourage the patient to talk with their physician and/or make an appointment if they have “red flags” that would suggest genetic counseling is indicated.
- Rarely have institutions reported that physicians routinely mention negative results to the patient and review whether a genetics appointment is warranted for other reasons (e.g. polyposis, strong family history, multiple primaries, early age at diagnosis).
Optimizing and Maintaining Tumor Screening
- Regardless of how well you plan, it is unlikely that you can anticipate everything.
- Most institutions we have talked to have altered their plans and this is to be expected.
- Maintaining records on how the plan was executed including changes that are made (and on what dates) is critical when evaluating the outcomes.
- It may also be useful to assess the timeliness of follow-up and results disclosure.
Reflecting and Evaluating to Ensure Quality
Importance of tracking
- Routinely tracking which patients have received screening and whether “screen positive” patients follow-up with germline genetic testing is critical for ensuring or improving successful implementation.
- Providing stakeholders with feedback (particularly when a patient is identified with Lynch syndrome) has helped others to promote ongoing support for the screening program and has helped to win over some individuals who were skeptical or hesitant to perform screening in the first place.
- Although few negative outcomes have been reported thus far, tracking any perceived negative outcomes is critical to making improvements or averting issues in the future.
Value of reflecting on the implementation process
- By discussing what seems to work and what does not, several centers have developed ways to streamline or improve their process.
- This should be done periodically, and whenever there are any changes in key personnel, challenges with patient follow-through, or any perceived negative outcomes.
Knowing if you are successful
- In addition to patient follow-through with genetic counseling and germline testing, other implementation outcomes have been measured at several different institutions and this data is relatively easy to collect and compare with expected outcomes based on data from large centers that have been doing screening for years.
- An excel spreadsheet has been created where you can track the number of screen positive patients you can expect, the number who will require counseling and germline testing (depending on your screening protocol), and the number of patients with Lynch syndrome who are expected to be identified. This can be used for quality control if the numbers are substantially different than expected. However, if volumes are low, numbers could differ from what is expected simply due to chance. [NOTE: This excel spreadsheet can also be a useful tool to anticipate counseling volumes when preparing to start a screening program].
Dealing with challenges that may arise with tumor screening
- A list of frequently asked questions for dealing with various challenges has been compiled.
- Consider signing up to be a member of LSSN (membership is free). As a member you can pose questions to the LSSN listserv or ask for feedback on challenges you may face.
Note: This guide was developed using the Consolidated Framework for Implementation Research (CFIR), review of the literature, and data from discussions with numerous stakeholders at organizations that have and have not implemented tumor screening.